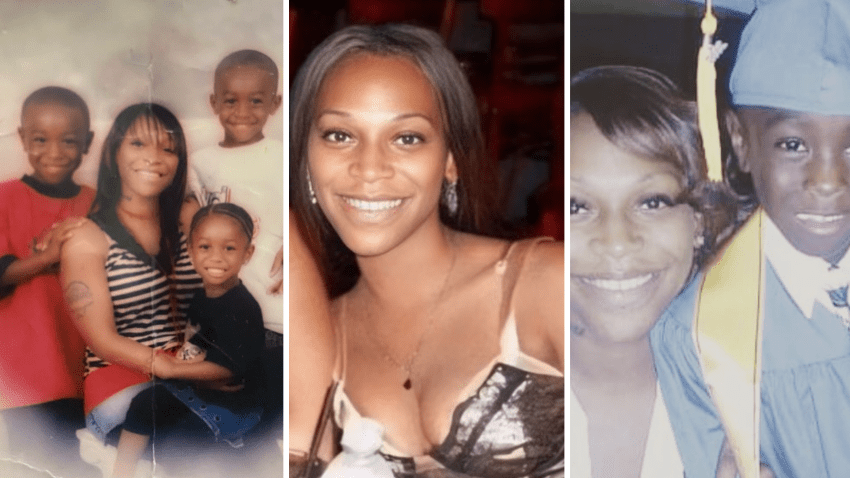‘We're still living': Man has a fresh start in Miami after leaving Haiti's turmoil
For one man, Haitian Heritage Month means a fresh start and a feeling of safety after recently moving from Haiti to the U.S. to escape the violence that’s taken over much of the country.
-

Agreement with City of Miami to end racial gerrymandering lawsuit over redistricting map
After a major ruling from a federal court came down in April regarding redistricting plans in the city of Miami, where the city’s voting map was tossed out, a new voting map has been approved by... -

Canadian man found unconscious while spearfishing off Key West dies
A Canadian man who was found unconscious while free-diving off Key West Tuesday has died, officials said.
-

Miami-Dade Police searching for man who allegedly fled hit-and-run that killed woman
Police are searching for a man accused of fleeing the scene of a crash that left a woman dead in northwest Miami-Dade over the weekend.
-

Trial begins for Kendall man accused of fatally shooting neighbor in 2015 dog poop dispute
Nearly nine years after authorities said a dispute over dog poop ended with a Kendall man fatally shooting his neighbor, the alleged gunman is finally on trial.